Public Investment, Private Gain:
Canada’s Role in the mRNA Vaccine Breakthrough
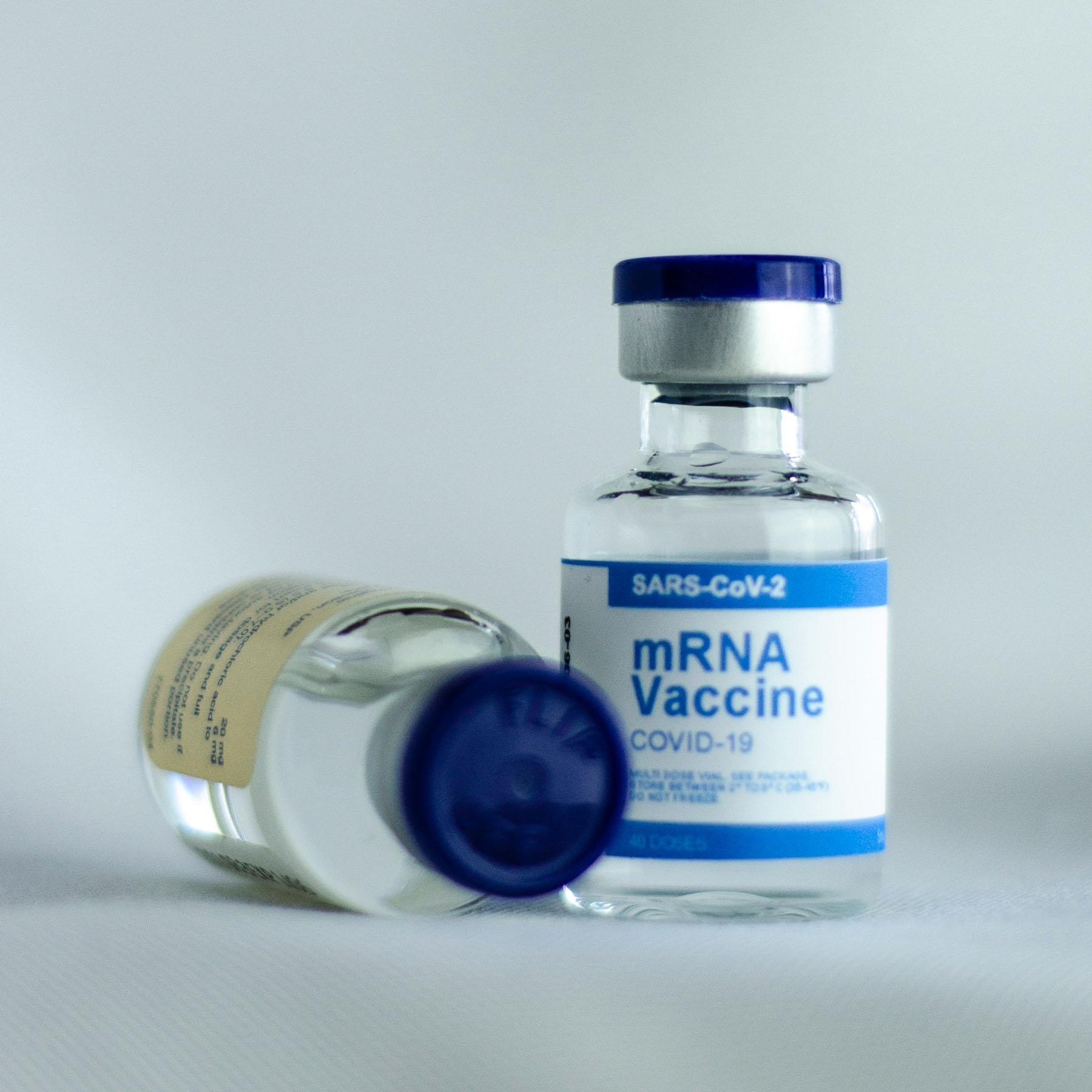
On January 30, 2020, the World Health Organization declared a public health emergency over the global outbreak of COVID-19. While Pfizer and BioNTech quickly gained recognition for their vaccine breakthrough, the critical role of Canadian science and innovation in enabling the vaccine’s success remains less acknowledged.
This case study examines Canada’s under-recognized yet foundational role in the development of the COVID-19 mRNA vaccines, specifically through a lipid nanoparticle (LNP) delivery system. This LNP system is a made-in-Canada innovation, stemming from research that originated at the University of British Columbia (UBC), and was fuelled by millions of dollars in federal grants and contributions.
Despite decades of public investment, Canada failed to secure access to the intellectual property (IP) underpinning this technology. When COVID-19 vaccines were urgently needed, Canada was forced to negotiate supply contracts from a position of weakness, with no domestic distribution rights or economic benefit from a technology it had helped create. This case highlights a gap in public policy regarding the absence of effective mechanisms to protect IP generated through publicly funded research.
Case Study #22
Download Includes: Case Study, Teaching Note
ISSN 2819-0475 • doi:10.51644/BCS022
Author
Research Themes
Health
Intellectual Property
Law
Public Policy